CALL 9463138669, ANAND CLASSES|BEST JEE MAINS ONLINE CHEMISTRY COACHING PREPARATION IN JALANDHAR PUNJAB

ANAND CLASSES offers Coaching Classes for IIT JEE Main in Urban Estate Phase-II Jalandhar. ANAND CLASSES is the prevalent establishment in the Jalandhar for Online IIT JEE Main Coaching preparation. It is surely famous for its selective class management classes and productive. Best Online IIT JEE Main Exam Coaching institute in Jalandhar. The faculty at ANAND CLASSES is highly qualified and vastly experienced in successfully coaching students for IIT JEE Main Exam. Download Hydrogen Spectrum NCERT Study Material pdf. Download Chemistry NCERT Study Material pdf. Best Chemistry Classes Jalandhar
Hydrogen Spectrum Introduction
We all know that electrons in an atom or a molecule absorb energy and get excited, they jump from a lower energy level to a higher energy level, and they emit radiation when they come back to their original states. This phenomenon accounts for the emission spectrum through hydrogen too, better known as the hydrogen emission spectrum.

In the late 1800s, it was known that when a gas is excited using an electric discharge and the light emitted is viewed through a diffraction grating; the spectrum observed consists not of a continuous band of light, but of individual lines with well-defined wavelengths. Experiments have shown that the wavelengths of the lines were characteristic of the chemical element emitting the light. They were an atomic fingerprint which resulted from the internal structure of the atom.
What is Hydrogen spectrum?
The hydrogen spectrum is an important piece of evidence to show the quantized electronic structure of an atom. The hydrogen atoms of the molecule dissociate as soon as an electric discharge is passed through a gaseous hydrogen molecule. It results in the emission of electromagnetic radiation initiated by the energetically excited hydrogen atoms. The hydrogen emission spectrum comprises radiation of discrete frequencies. These series of radiation are named after the scientists who discovered them.
Hydrogen spectrum wavelength
When a hydrogen atom absorbs a photon, it causes the electron to experience a transition to a higher energy level, for example, n = 1, n = 2. When a photon is emitted through a hydrogen atom, the electron undergoes a transition from a higher energy level to a lower, for example, n = 3, n = 2. During this transition from a higher level to a lower level, there is the transmission of light occurs. The quantized energy levels of the atoms, cause the spectrum to comprise wavelengths that reflect the differences in these energy levels. For example, the line at 656 nm corresponds to the transition n = 3 n = 2.

Hydrogen emission spectrum:
In the year 1885, on the basis of experimental observations, Balmer proposed the formula for correlating the wave number of the spectral lines emitted and the energy shells involved. This formula is given as:
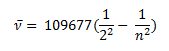
This series of the hydrogen emission spectrum is known as the Balmer series. This is the only series of lines in the electromagnetic spectrum that lies in the visible region. The value, 109,677 cm-1, is called the Rydberg constant for hydrogen. The Balmer series is basically the part of the hydrogen emission spectrum responsible for the excitation of an electron from the second shell to any other shell. Similarly, other transitions also have their own series names. Some of them are listed below,
- Transition from the first shell to any other shell – Lyman series
- Transition from the second shell to any other shell – Balmer series
- Transition from the third shell to any other shell – Paschen series
- Transition from the fourth shell to any other shell – Bracket series
- Transition from the fifth shell to any other shell – Pfund series

Johannes Rydberg, a Swedish spectroscopist, derived a general formula for the calculation of wave number of hydrogen spectral line emissions due to the transition of an electron from one orbit to another. The general formula for the hydrogen emission spectrum is given by:
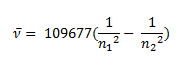
Where,
n1 = 1,2,3,4 …
n2 = n1 +1
ν= wave number of electromagnetic radiation. The value 109,677 cm-1 is known as Rydberg constant for hydrogen.
To learn more about hydrogen emission spectrum download ANAND CLASSES – The Learning App.
—————————————————————————————————————————
ANAND CLASSES offers IIT-JEE Mains Entrance Exam Coaching in Urban Estate Phase-II Jalandhar. ANAND CLASSES is the prevalent establishment in the Jalandhar for IIT-JEE Mains Entrance Exam. It is surely famous for its selective class management classes and productive Best IIT-JEE Mains Entrance Exam coaching institute in Jalandhar.
The faculty at ANAND CLASSES is highly qualified and vastly experienced in successfully coaching students for IIT-JEE Mains Entrance Exam.
ANAND CLASSES is known as leading institute for preparation of IIT-JEE Mains Entrance Exam Coaching in Jalandhar. We at ANAND CLASSES provide coaching for IIT-JEE Mains Entrance Exam from the past 15 years. The IIT-JEE Mains Entrance Exam Coaching Center in Jalandhar, Punjab.
Fresh batches for IIT-JEE Mains Entrance Exam are going to start. ANAND CLASSES Coaching Institute is the only well known coaching institute in Jalandhar that provides coaching for IIT-JEE Mains Entrance Exam. At ANAND CLASSES Coaching Institute, we constantly strive to improve our teaching methodology, study material and classroom assignments. The Course Methodology is dynamic as it takes into account the changes that we notice in the pattern of the examination.
ANAND CLASSES is a professionally managed and organized IIT-JEE Mains Entrance Exam coaching centre in Jalandhar, offering best coaching and preparing the job aspirants for Graduate Students.
ANAND CLASSES is the best coaching center for IIT-JEE Mains Entrance Exam.
To be learn Rutherford Atomic Model Click here :
To be learn Atomic Structure Click here :
To be learn Isotopes & Isobars Click here :
Isotopes and Isobars | Difference between isotopes and isobars
To be learn Milliken’s Oil Drop Experiment Click here :
